Medistri’s Biocompatibility Testing for Medical Devices impacts healthcare companies in more than 20 countries around Europe.
The company’s laboratory provides Biocompatibility Testing for Medical Devices according to ISO 10993. Working primarily with customers across Europe in Austria, Belgium, Finland, France, Germany, Greece, Ireland, Italy, Norway, Denmark, Israel, Switzerland, Luxembourg, Croatia, the Netherlands, Portugal, Hungary, and the UK.
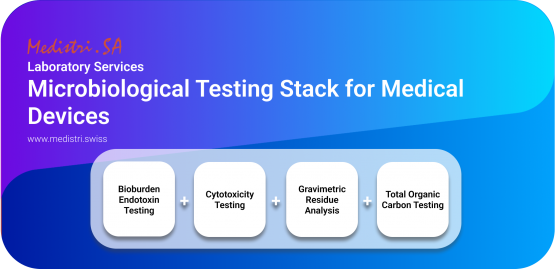
Microbiological Testing Stack for Medical Devices
Developing innovative medical device technology is a high-risk operation.
That’s why start-up ventures to large established medical devices manufacturers use Medistri’s laboratory testing services to deliver accurate and reliable results for all their testing needs.
[NEW] PDMS Analysis by FTIR
PDMS analysis by FTIR is a test that consists of extracting and identifying PDMS residues in a qualitative/semi-quantitative method in pharmaceuticals or medical samples.
Cytotoxicity Testing — ISO 10993–5
Cytotoxicity testing is used to determine the toxicity of medical devices and materials. Cytotoxicity testing evaluates whether a material can affect living cells. Medical Device Manufacturers are required to show that their devices are not cytotoxic.
Biocompatibility Testing - ISO 10993
Biocompatibility is defined as “The ability of a device material to perform with an appropriate host response in a specific situation”.
Volatile Organic Compounds (VOCs) Analysis by GC/MS
ISO 10993-18 focus on chemical characterisation of medical device material within a risk management process. Part of this process is the identification, quantification of extractables and leachable compounds using gas chromatography/mass spectrometry (GC/MS) technology.
GC/MS Analysis
GC/MS Analysis (also called Gas Chromatography/ Mass Spectrometry) is an analytical process that utilises the capabilities of Mass Spectrometry and Gas Chromatography in order to determine the chemical compounds within a sample.
FTIR Spectroscopy Analysis
The Fourier Transforms converts the raw data from the infrared emission into a spectrum which Medistri’s laboratory uses to study, analyse and identify the compound.
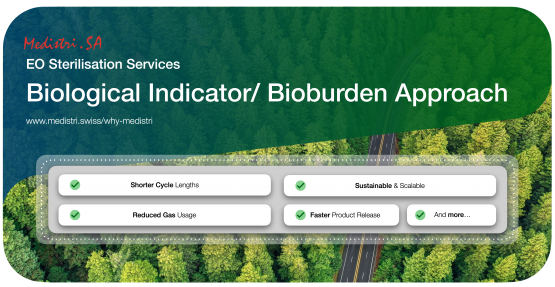
Designing Sustainable & Scalable EO Sterilisation Processes
At Medistri, we believe our focus on using our resources to create sustainable solutions are both ambitious and necessary.
We've popularised & offered a smarter alternative to the market called the “ Biological Indicator/ Bioburden Approach”.
Pagination
- First page
- Previous page
- 1
- 2
- 3
- 4
- 5
- 6
- 7