Our laboratory
Medistri's in-house laboratory is a full-service contract laboratory, specialised in microbiology, analytical, bioanalytical chemistry & cell biology services, custom synthesis, and R&D.
Our laboratory works according to ISO 17025 (current version) and is accredited since 2008 by the Swiss Accreditation Service (SAS). All testing can be performed according to European or US pharmacopeias.


Areas of Competence
Biocompatibility
An integral part of biological risk assessment, biocompatibility testing assesses the compatibility of medical devices with a biological system.
Chemical Characterisation
Determination of the composition or purity of your materials, quality control and identification of unknown substances.
Cytotoxicity
Determine the toxicity of compounds on cells in a qualitative or quantitative manner.
Toxicological Studies
Toxicology studies are used to characterise the toxicity profile of a drug by identifying its impact on organ structure and/or functionality. This includes assessment of the severity and reversibility of toxicity, as well as dose ranges and their relationship to exposure.
Heavy Metal Elemental Analysis
For pharmaceuticals, heavy metal loading quantification is performed to identify contaminants and to perform bioanalysis on therapeutic products designed to contain heavy metals.
Elemental and Trace Metal Analysis
Measuring inorganic impurities in pharmaceutical, biological and nutraceutical products.
Medical Device Shelf Life Testing
Medical device manufacturers wishing to collect shelf life data on their products may subject their devices to long-term stability studies or accelerated ageing studies. Many different criteria can be used to assess the shelf life of a medical device, including sterility or packaging integrity. It is therefore important that the criteria and test methodology are decided before testing begins.
Small Molecule Identification and Purity Testing
Identity and purity testing are crucial requirements for product release. There are a variety of testing methods for determining pharmaceutical substances and products to ensure that the final pharmaceutical products meet the customer's specifications. In particular, infrared spectroscopy is the most powerful test for confirming the identity of small molecules and drug products. As stated in the USP <197>, "the IR absorption spectrum of a substance [...] provides perhaps the most conclusive evidence of substance identity that can be achieved from a single test". FTIR results can be used to compare several specific peaks in the IR spectrum with those of a reference standard, thus establishing solid acceptance criteria.
Extractable’s & Leachable’s
The closure systems of containers (vials, syringes, bottles for commercial products etc.) intended for use in pharmaceutical applications could contribute significantly to the increase of impurities in the final product. Therefore, chemical characterisation of Extractables and Relargables (E/R) should be considered to identify the substances and components that generate them. The testing of extractables and leachables (E&L) is therefore crucial for the release of the product.
Residual Testing
Analyse the chemical residues on your medical device to provide insight into the cleaning operations after production. We perform gravimetric analysis to determine the total amount of residue coming off the device. We can use GC and GC-MS to quantify residual chemicals left behind by detergents and cleaning agents and UV-Vis spectrophotometry to measure residual proteins. We can analyse total organic carbon (TOC) to quantify organic carbon residues on the device left by oils, detergents and adhesives.
Sterile Barrier Integrity Testing
Packaging integrity testing is used to detect packaging problems that could affect the sterility of a medical device. Sterile products may be subjected to environmental stress to simulate the extreme conditions a product might encounter during shipment or storage. The product packaging is then subjected to a microbial challenge or dye penetration test to determine whether it has retained its microbial barrier properties.
Bioburden Testing
Many single-use medical devices are sterilised using EtO, steam or radiation technologies. Manufacturers are required to validate the sterilisation process and usually require bioburden and sterility testing.
Plastics Testing
Manufacturers benefit from material classification if plastic resins are likely to be used in their medical devices. A plastic material that has passed USP Class Plastics testing should be more likely to produce favourable biocompatibility results.
Environmental Monitoring
Whether you are manufacturing a medical device or a drug API, the safety of end users is at stake. Implementing a comprehensive environmental monitoring programme will allow you to know the state of your environment, giving you the means to control it and make changes if necessary. The Medistri team can help you strengthen your quality management system and can perform a wide range of testing methods to accurately monitor the environmental conditions of your manufacturing process.
LAL & Bacterial Endotoxin
The LAL (Limulus Amebocyte Lysate) test, also known as the bacterial endotoxin test, is an in vitro test used to detect the presence and concentration of bacterial endotoxins in drugs and biologicals, and is an important part of pharmaceutical microbiology.
Sterility Tests
Sterility testing and the development or validation of product-specific methods are critical steps in the drug development process to ensure that viable contaminating micro-organisms are not present in a product.
Focused Excellence
Pharmaceutical Industry
The largest pharmaceutical companies in the world use Medistri's laboratory infrastructure to focus on comprehensive, innovative and fast solutions to optimise operations.
MedTech Industry
Start-up ventures to large established medical devices manufacturers use our laboratory services to deliver accurate and reliable results for all their testing needs.
BioTech Industry
Medistri provides laboratory services a focused set of solutions tailored to be integrated within the unique and specific needs of early-stage Biotechnology companies.
Better relationships, better prospects
Whether you are a start-up or a large company, whether you choose a standard protocol test or request a fully customised test method, Medistri's scientific advisors will ensure that your specific needs are met to the highest standards. We ensure that your team is informed and regularly updated with detailed results throughout all stages of your project or analysis.
Our laboratory team gathers all the data, presents the results and recommends risk management proposals in a transparent, detailed and accurate manner.
Efficient testing systems
From the structure of the management team, to the creation of new types of tests, to the continuous investments we make in our laboratory infrastructure, Medistri is dedicated to finding solutions to our customers' most complex challenges.
Our laboratory is located in the heart of Switzerland, allowing us to continuously serve the country's most innovative universities, research institutes, start-ups and large companies.
The resources combined with the expertise of our laboratory, sterilisation and quality teams enable our customers to integrate their product development into their existing operational workflow.
State-of-the-Art
Our customers are focused on finding solutions to the healthcare industry's most complex problems. We've designed our facilities around helping them find these solutions.
Since our foundation, we've aligned ourselves with our customers and allocated our resources towards combining the best intellectual resources with the most sophisticated instruments.
Modern expertise:
- Continuous development of Test Methods
- Continuous training on technical development
- Continuous education on regulatory changes and complexities.

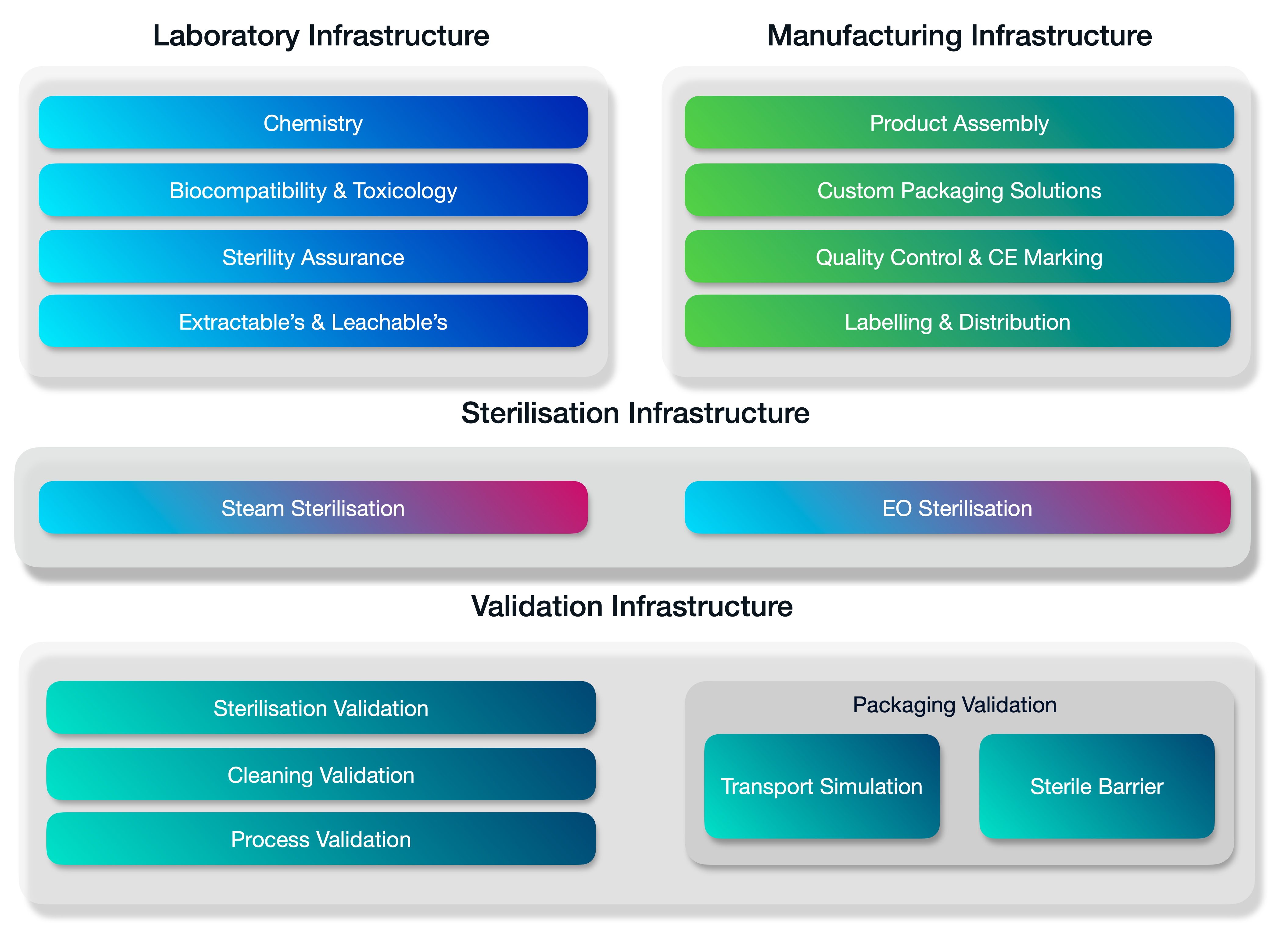
A fully integrated stack of services.
Medistri is continually innovating our in-house range of services. We’re expanding our services to offer a complete integrated suite of solutions for innovative healthcare companies. Organisations of every size — from startups to large enterprises use our suite of services to grow & optimise their business.
Medistri combines all its technical infrastructure and places quality at the heart of our day-to-day operations. Allowing you to simplify your supply chain management and focus on growth.
Discover how our customers use Medistri's stack of integrated services ➝
Laboratory Services for Innovative Ideas
Powerful laboratory infrastructure to help the world's most innovative healthcare companies.
Use our suite of services and expertise to seamlessly develop your most complex ideas.
Are you ready to get started?
Contact us and our qualified team will respond.