Customized Assembly for Startups
At Medistri, we specialize in the precision-driven assembly, packaging, and sterilization of medical devices or pharmaceutials, catering to both emerging startups and established companies. Our process is designed to support the unique needs of each client, from prototype development to routine large-scale production.
Medistri’s Logistics Heatmap Tool
At Medistri, we’re always exploring new ways to make your logistics planning smoother and more efficient. That’s why we’re excited to introduce the Logistics Heatmap Tool - an innovative solution designed to provide you with valuable insights into your supply chain. With this tool, you can make more informed decisions and optimize your logistics strategy, all by simply looking at historical data that is visualized in an easy-to-understand format.
EO Sterilisation & Packaging
The design of a medical device’s packaging plays a critical role in ensuring the product’s success and safety. It should be developed during the early stages of the device’s creation to avoid costly challenges later. Packaging is not just a protective layer; it serves as a sterile barrier that safeguards the medical device from manufacturing to final use.
Medistri Expands to Hungary: Strengthening Our Commitment to Healthcare
Medistri is proud to announce the opening of our new facility in Székesfehérvár, Hungary, marking a significant milestone in our mission to enhance global healthcare solutions.
Medistri’s ISO 13485 Certification
The implementation of a quality management system is a cornerstone for the development and delivery of safe, effective medical devices. ISO 13485 certification ensures that every aspect of the production process aligns with international standards for quality and safety. At Medistri, this certification reflects our commitment to supporting the medical device industry by maintaining the highest levels of precision and compliance in our processes, ensuring that patient safety is never compromised.
Packaging Optimisation for Sterilisation
The design of a medical device’s packaging is a critical component that should be addressed during the early stages of the product’s development phase. Packaging plays a vital role not only in maintaining the sterility of the medical device but also in ensuring its safe delivery from the manufacturing process to the point of use with patients. This makes it essential to consider packaging as a priority from the outset.
Ethylene Oxide (EO) and Steam Validation - A Comparative Overview
Sterilization validation is the process of ensuring that a sterilization method consistently achieves the desired level of sterility, as defined by international standards. This involves validating each parameter of the sterilization cycle—temperature, time, pressure, and for EO, gas concentration and humidity—ensuring that the sterilization process is effective, reproducible, and compliant.
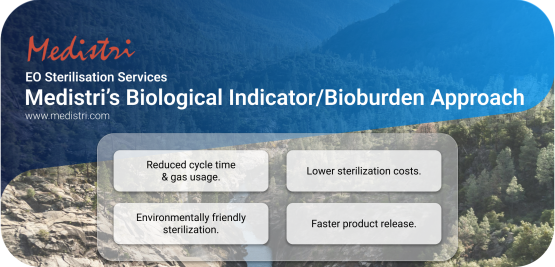
Medistri’s Biological Indicator/Bioburden Approach
At Medistri, we are committed to creating sustainable solutions by focusing on scalable innovation. We aim to drive progress through new technologies, financial structures, and renewable energy deployment. In addition to our gas treatment technology, we have invested resources to offer a smarter alternative to the traditional sterilization methods. One of our key innovations is the "Biological Indicator/Bioburden Approach," a more efficient and environmentally friendly alternative to conventional sterilization.
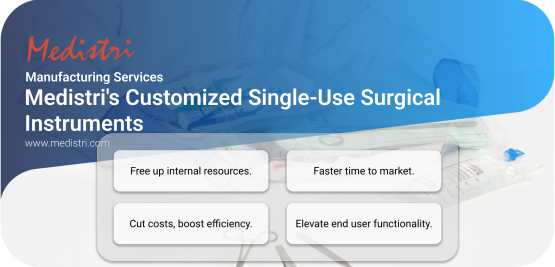
Medistri's Customized Single-Use Surgical Instruments
Created for start-up companies and early-stage products, Medistri's manufacturing team can offer primary, secondary, and tertiary packaging solutions that fit your strategic & regulatory requirements.
Medistri's Medical Device Sterilisation
Medical Devices Sterilisation is essential to prevent infections and protect patient health. Adhering to standards like ISO 11135 for EO sterilisation ensures rigorous validation and controls, guaranteeing sterility and maintaining the highest levels of safety and reliability.